Abstract
Background/introduction: The effectiveness of Bcl-2 inhibitors as a treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) was established by the approval of venetoclax in pts with CLL/SLL across all lines of therapy. However, the related adverse events (AEs) and emergence of BCL2 mutations, resulting in resistance, can limit the utility of venetoclax. BGB-11417 is a highly selective Bcl-2 inhibitor with potency >10 times that of venetoclax in biochemical assays. BGB-11417 monotherapy is tolerable, with no maximum tolerated dose (MTD) reached after dose escalation through all planned doses to 640 mg once daily (QD) in pts with non-Hodgkin lymphoma (EHA 2022. Abstract P687).
The combination of Bcl-2 and Bruton tyrosine kinase (BTK) inhibitors is tolerable with synergistic activity in CLL and mantle cell lymphoma (MCL) (J Clin Oncol 2019;37:2722-9; N Engl J Med 2019;380:2095-103; EHA 2020. Abstract S158; N Engl J Med 2018;378:1211-23). ZANU, a next-generation BTK inhibitor, has shown favorable activity and safety in pts with CLL/SLL (EHA 2021. Abstract LB1900) and Waldenström macroglobulinemia (Blood. 2020;136(18):2038-2050). BGB-11417-101 is an ongoing first-in-human phase 1/1b dose-escalation/expansion study (NCT04277637). Pts with various B-cell malignancies were enrolled; data from CLL/SLL cohorts are presented here.
Methods: In separate monotherapy and combination therapy cohorts, pts received escalating doses of BGB-11417 (40, 80, 160, 320, or 640 mg QD) with a ramp-up to the intended target dose to minimize risk of tumor lysis syndrome (TLS). In combination therapy cohorts, pts received ZANU (320 mg QD or 160 mg twice daily) beginning 8-12 weeks before BGB-11417. Dose-limiting toxicity for each cohort was evaluated by a Bayesian logistic regression model during dose ramp-up through day 21 at the intended dose. AEs were reported per Common Terminology Criteria for AEs v5.0. Minimal residual disease (MRD) was assessed by a European Research Initiative on CLL flow cytometry assay.
Results: As of May 15 2022, 50 pts with CLL received treatment: 6 monotherapy (all relapsed/refractory [R/R]) and 44 combination (22 R/R; 22 treatment naïve [TN]). The monotherapy CLL cohort received BGB-11417 doses up to 160 mg. Based on emerging safety data from other cohorts, pts in combination cohorts with R/R CLL received BGB-11417 up to 640 mg and pts with TN CLL received up to 320 mg (data include 8 pts in ZANU pretreatment not yet dosed with BGB-11417). MTD has not yet been reached for any CLL cohort, with dose escalation ongoing. Median follow-up was 11.5 mo (range 8.5-18.3) for monotherapy and 5.8 mo (range 0.2-10.5) for combination.
Treatment-emergent AEs (TEAEs) across all doses are listed in the Table. With monotherapy, cytopenias were the most common TEAEs (≥50%), with 33% grade ≥3. With combination, contusion, neutropenia, and low-grade gastrointestinal toxicity were the most common TEAEs (≥22.7%); neutropenia was the most common grade ≥3 TEAE (11.4%) with 5 pts. No pts discontinued monotherapy treatment, and 1 pt discontinued combination treatment (disease progression; Richter transformation). Only 1 high-risk pt with CLL on monotherapy had laboratory TLS that resolved with no intervention (overall laboratory TLS ≤2%). No clinical TLS was reported. Diarrhea was mostly grade 1 and grade ≥3 was not seen.
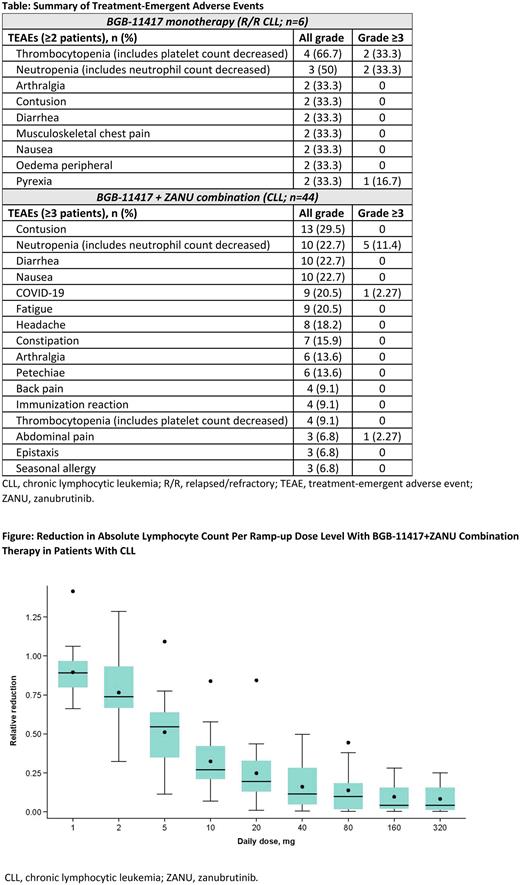
Although efficacy data are early, most pts with CLL/SLL had notable reductions in absolute lymphocyte count (ALC) with responses seen at doses as low as 1 mg (Figure), consistent with improved potency of BGB-11417 vs venetoclax. Four responses (66%, partial response [PR] or better) and 32 responses (72.7%, PR with lymphocytosis or better) were observed with mono- and combination therapy, respectively. MRD data are early: among 4 MRD evaluable pts at 160 mg, 3 pts (2 monotherapy; 1 combination) had a peripheral blood CLL count <10-4 at 24 weeks after BGB-11417 initiation.
Conclusion: These preliminary data show BGB-11417, alone or in combination with ZANU, was well-tolerated in most patients. Grade ≥3 neutropenia was uncommon and manageable. Efficacy is supported by the rapid reduction in ALC during ramp-up, and early response data are promising. TLS rates are low; the prophylactic measures and ramp-up schedule seem to adequately mitigate TLS across all dose levels tested. Mature MRD data are forthcoming, and venetoclax-treated CLL/SLL cohorts will soon be open for enrollment.
Disclosures
Cheah:AbbVie: Research Funding; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tam:LOXO: Honoraria; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria; Beigene: Honoraria, Research Funding. Lasica:Janssen: Other: Education. Verner:Janssen Cilag Pty Ltd: Research Funding. Browett:Arrowhead: Honoraria; BeiGene: Research Funding; MSD: Honoraria; Roche: Research Funding; Abbvie: Honoraria; Eysa Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Anderson:Gilead: Honoraria; CSL: Honoraria; The Walter and Eliza Hall Institute: Current Employment; Novartis: Honoraria; Takeda: Honoraria; Janssen: Honoraria; AbbVie: Honoraria; AstraZeneca: Honoraria. Hilger:BeiGene: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Simpson:BeiGene: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Travel, Accommodations, Expenses. Opat:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal